FOLLOW US

Stay informed, follow us at
@UnderstandAML
Understanding AML: Remission & Relapse
Understanding AML:
Remission & Relapse
Even when morphological remission is achieved, patients with acute myeloid leukemia (AML) are still at risk of relapse.1,2
See one AML patient's experience with remission and relapse, featuring insights from AML expert, Dr DiNardo.
Learn how AML thought leaders are advancing our understanding of remission and relapse.
AML, acute myeloid leukemia.

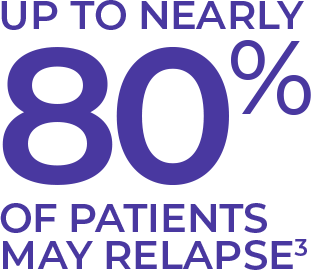
Although responses to induction therapy are generally high in AML,
responses can be short-lived and most patients relapse.3,4
Experts take a new look at a challenging problem.
HEAR THE EXPERT:
AN AML DISCUSSION
These audio clips and transcripts are excerpts from a paid interview with Dr Richard Stone, sponsored by Bristol Myers Squibb. These are the opinions of Dr Richard Stone and not the views of Bristol Myers Squibb.
These are the opinions of Dr Richard Stone and not the views of Bristol Myers Squibb.
Most patients are going to relapse—even if they have a stem cell transplant, some will relapse. So, I think it’s just intrinsically disease.
It is a clonally complex disease, so therapy may be able to get rid of 99.9 percent of the cells but we have that one lurking clone that can’t be killed by therapy.
That’s very important. Complete remission in AML is generally thought to be necessary but not sufficient to achieve a cure. If the goal in this, let’s say, younger patient is cure, which it generally always is in younger patients and sometimes even in older patient but anyway if cure is the goal, remission of some sort is necessary to get to that goal.
Far and away the most important reason why people who achieve remission don’t survive normal natural history is because they relapse after the consolidation therapy. And why do they relapse? They relapse because they have some clones, presumptively, or cells just are not completely enough eradicated by the initial therapy and that’s where the rubber meets the road.
MRD is alternatively called minimal residual disease, but most of us prefer to call it measurable residual disease because we’ve always measured it. We’ve measured it with a bone marrow test that was fairly not sensitive. Now we can measure it by asking whether the immunophenotypic clone that was present at diagnosis is still present at the time of morphological remission.
Of those in remission, probably about half have an MRD-positive remission where they can still have detectable disease at the time of this morphologic remission. And that’s a tough—it’s all well and good to be able to detect them but the real problem is what the heck do we do about it once we see it?
These are the opinions of Dr Richard Stone and not the views of Bristol Myers Squibb.
I stand by that statement and I think we can learn a little bit, maybe, from ALL in this regard. So what we need is an effective MRD eraser, I think, to change people from detectable disease to undetectable disease. I mean, there’s a lot of leaps here.
Our cutoff for morphological disease is still less than 5 percent blasts. I already mentioned that’s just part of the equation—even if we’re just looking at things like the bone marrow and the blood counts because we still want full hematopoiesis if possible. If we get partial restoration of hematopoiesis, it probably means the remission is not as deep if you can measure the actual leukemic burden, although we don’t know that for certain and it seems likely, so evolution of morphological remission hasn’t changed very much in all those years.
If you’re more of a guy who likes to keep their eyes open, then you should probably measure it because we’re learning about what to do about it. It does have prognostic information which might be helpful to patients.
The problem is the techniques themselves are not standardized in the US and not easily measured for logistical reasons. So it’s a tricky, tricky question. I still recommend that they be measured but what to do about it is very controversial. I guess I would try to marry the upfront prognostic data with this outback after two cycles of chemotherapy MRD data and try to figure out what the heck we should do with our patients. But we have to acknowledge that it’s far from set in stone.
These are the opinions of Dr Richard Stone and not the views of Bristol Myers Squibb.
That’s the problem. The answer is there is no level that’s reassuring enough because even people with MRD-negative disease...some of the people with MRD-negative disease still will relapse. And that’s simply because we don’t measure every last leukemic cell. We measure a lot more leukemic—or we can pick up a lot more leukemic cells by using one of these new techniques but it doesn’t—it’s not perfect. It’s just a matter of odds if you have MRD-negative disease you’re less likely to relapse. That’s it.
So, takeaway number one, it’s probably going to be a very important and routinely measured issue soon, once we standardize the technique. And, number two, because PCR is a Next Gen sequence that’s kind of patient specific and if you can do flow cytometry you can apply it to almost everybody. I suspect in the most commonly used technique will be flow cytometry. Three, and the most important thing, is that at the moment we don’t know what—we know that it’s better to not have measurable disease by a sensitive technique but we don’t know what to do with it yet in AML. And we’re learning about that. All of us would like to get rid of the residual disease and hopefully that will lead to a better natural history.
VIEW SLIDE DECK
MRD as a Predictor of Relapse in AML
A slide presentation covering remission and relapse in AML and the importance of detecting and monitoring MRD.
EXTERNAL AML-RELATED ARTICLE
Very poor long-term survival in past and more recent studies for relapsed AML patients: The ECOG-ACRIN experience
References: 1. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-447. 2. San Miguel JF, Martínez A, Macedo A, et al. Immunophenotyping investigation of minimal residual disease is a useful approach for predicting relapse in acute myeloid leukemia patients. Blood. 1997;20(6):2465-2470. 3. Meyers J, Yu Y, Kaye JA, Davis KL. Medicare fee-for-service enrollees with primary acute myeloid leukemia: an analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl Health Econ Health Policy. 2013;11(3):275-286. 4. Luskin MR, Stone RM. Can minimal residual disease determination in acute myeloid leukemia be used in clinical practice? J Oncol Pract. 2017;13(8):471-480.
You are now leaving
UnderstandingAML.com
and going to a third-party website outside of the control of Bristol Myers Squibb.
Click OK to proceed.






